ROI-C® Cervical Cage With VerteBRIDGE® Plating Technology
ROI-C featuring VerteBRIDGE plating is a zero-profile, stand-alone* cervical cage, with streamlined instrumentation and surgical technique, requiring minimal exposure.

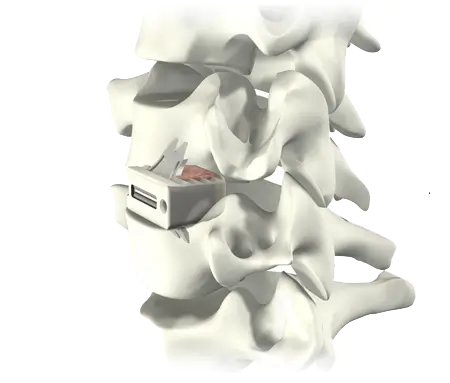
Elegance in Simplicity
ROI-C with VerteBRIDGE plating offers a sleek and minimalistic stand-alone solution. Streamlined instrumentation functions in-line with the disc space, requiring minimal exposure, while an innovative implant design leaves minimal hardware in the patient, all without sacrificing stability.
System Features
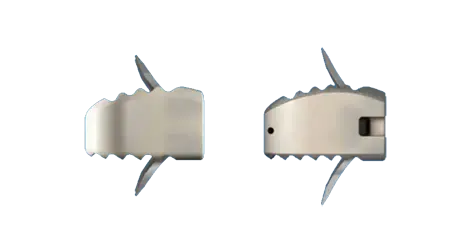
Simplicity
- Ease of use: the ROI-C surgical technique requires fewer steps than a traditional ACDF (with plate and screws).
- A mini open technique allows for less retraction.ROI-C with VerteBRIDGE offers a complete solution for an anterior cervical fusion.
- No hardware whatsoever protrudes anterior of the vertebral body.

Confidence
- With two geometries and more footprints than any other competitive product, surgeons can select the perfect fit for any anatomy. PEEK® and Titanium-coated PEEK® accommodate surgeon choice and preference.
- Streamlined instrumentation alleviates challenges of patient anatomy such as the chin.
- Large fit and fill of available footprints provides initial stability, while self-locking plates are designed for initial and long-term stability.
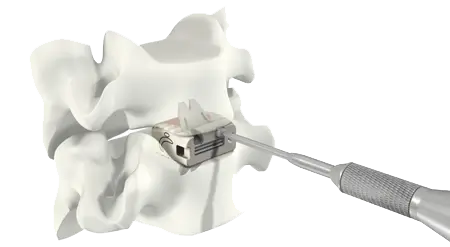
Experience
- Over 80,000 implantations performed using VerteBRIDGE plating.
- Biomechanical testing demonstrates that ROI-C with VerteBRIDGE plating performs at a similar level to other products on the market.
- ROI-C with VerteBRIDGE plating is the stand-alone* market leader.
Specifications
ROI-C Sizing Options
Four Footprints
ROI-C Plates
ROI-C Plates Three Locking Mechanisms
Related Products
HIGHRIDGE's anterior cervical plate products are engineered to treat complex cases with clinical effectiveness, always with a commitment to patient safety.
Resources
Brochure & Surgical Technique
Important Information
Description
Description
The ROI-C Implant System ROI-C Titanium- Coated Implant System consist of ‘D’ shaped blocks in a variety of footprints and heights. The lordotic shape of the ROI-C Lordotic Implants & ROI-C Lordotic Titanium-Coated Implants allow for optimum surface area contact with vertebrae that embody a flat surface morphology. The curved shape of the ROI-C Anatomic Implants & ROI-C Anatomic Titanium-Coated Implants allow for optimum surface area contact with vertebrae that embody a curved surface morphology. The ROI-C Implant System & ROI-C Titanium-Coated Implant System are offered in a closed graft space design. The implants feature an enclosed chamber intended to be filled with autologous or allogenic bone graft composed of cancellous and/or corticocancellous bone graft. The superior and inferior surfaces of the implants have a pattern of teeth to provide increased stability and to help prevent movement of the device.
The ROI-C Titanium-Coated Implant System offers a porous plasma sprayed Titanium Coating of superior and inferior surfaces of the implants.
The ROI-C Implant System & ROI-C Coated Implant System are intended to be implanted singularly via an anterior approach and is intended to be used with autologous bone graft. The devices must be used with supplemental internal fixation. The ROI-C Implant System & ROI-C Titanium-Coated Implant System have been designed to be compatible with optional supplemental fixation specific for the system. The two piece VerteBRIDGE Anchoring Plate is available and may be used to affix the ROI-C Implant System & ROI-C Titanium-Coated Implant System implants to the underlying vertebral bone, and to specifically allow for the option of a standalone construct. Additional or other supplemental fixation may be used, as patient needs dictate.
Indications and Contraindications
Indications
The ROI-C Implant System & ROI-C Titanium-Coated Implant System are indicated for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one disc level from C2–T1. DDD is defined as discogenic pain with degeneration of the disc confirmed by history and radiographic studies. These patients should have had six weeks of nonoperative treatment. The ROI-C Implant System & ROI-C Coated Implant System implants are to be used with autogenous or allogenic bone graft composed of cancellous and/or corticocancellous bone graft and implanted via an open, anterior approach. Supplemental internal fixation is required to properly utilize this system.
Contraindications
Contraindications include, but are not limited to:
- Presence of fever or acute, chronic, systemic, or localized infection.
- Metal sensitivity or allergies to the implant materials, documented or suspected.
- Severe osteopenia.
- Pregnancy.
- Prior fusion at the level(s) to be treated.
- Patients unwilling or unable to follow post-operative care instructions.
- Other medical risks, anesthetics risks, or surgical conditions which would preclude the potential benefit of spinal implant surgery.
- Any condition not described in the indications for use.
Potential Side Effects
The list of side effects below is not exhaustive. The listed side effects are possible side effects known to potentially occur for procedures that may involve this type of device and surgical approach. These side effects can sometimes necessitate further surgical treatment.
- Fissure or fracture of implant components;
- Loss of fixation, dislocation and/or migration;
- Neurological complication, paralysis, soft tissue lesions, pain due to the surgical procedure, and/or breakage deformation;
- Injury to vessels, nerves and organs;
- Neurological and spinal dura matter lesions from surgical trauma;
- Superficial or deep-set infection and inflammatory phenomena;
- Venous thrombosis, pulmonary embolism and cardiac arrest;
- Hematoma and impaired wound healing.
LDR SPINE USA cannot be held responsible for any complications arising from: incorrect diagnosis, choice of incorrect implants or operating techniques, limitations of treatment methods, inadequate asepsis, any change in the product after delivery, incorrect handling before, during and after the surgery.
New side effect from materiovigilance MAUDE: Electric Arc with a bovie.
Warnings and Precautions
Warnings
- Risks associated with general surgery, orthopedic surgery, and the use of general anesthesia should be explained to the patient prior to surgery. It is also recommended that the advantages and disadvantages of surgery, the implants, as well as alternative treatment methods be explained to the patient.
- Potential risks associated with the use of this system, which may require additional surgery, include device component failure (bending, loosening or fracture), loss of fixation, non-union, fracture of the vertebra, neurological injury, vascular or visceral injury, neurological complications, over-distraction, trauma to nerve root or dura, incorrect implant positioning, implant migration, pseudoarthrosis, disc height loss (impaction of implant into vertebral end plates), allergy or inflammation, general adverse effects related to surgical procedures (e.g. anesthesia, infection), subsidence, expulsion.
- The device can break if it is subjected to increased loading associated with delayed union or non-union. If healing is delayed or does not occur, the implant could eventually break due to material fatigue. Factors such as the patient weight, activity level, and compliance to weight bearing or load bearing instructions, have an effect in the stresses to which the implant may be subjected, and may affect the longevity of the implant.
- Patients with previous spinal surgery at the level(s) to be treated may have different clinical outcomes compared to those without a previous surgery.
- Discard all damaged or mishandled implants.
- Under no circumstances may the implants be re-used. Although the device may appear intact on removal, internal modification due to the stress and strains placed on it, or small defects may exist which may lead to fracture of the implant.
- Implants removed from a patient that contact bodily tissues or fluids should never be reused at risk of contamination of the patient.
- Mixing Metal: Some degree of corrosion occurs on all implanted metal and alloys. Contact of dissimilar metals (e.g. stainless steels and titanium), however, may accelerate this corrosion process. The presence of corrosion may accelerate fatigue fracture of implants and the amount of metal compounds released into the body system may also increase. Internal fixation devices such as rods, connectors, screws, hooks, etc, which come into contact with other metal objects must be made from like or compatible metals. This is an important consideration when using supplemental fixation, as required by the indications for use of the System.
- Because different manufacturers employ different materials, varying tolerances, manufacturing specifications, and differing design parameters, components of the ROI-C Implant System & ROI-C Titanium-Coated Implant System should not be used in conjunction with components from any other manufacturer’s implant systems. Any such use will negate the responsibility of Zimmer Biomet Spine (formerly LDR Spine USA) for the performance of the resulting mixed component implant.
- Any decision by a surgeon to remove the implanted device should take into consideration such factors as the risk to the patient of the additional surgical procedure as well as the difficulty of removal.
- Implant removal should be followed by adequate postoperative management to avoid fracture.
Precautions
- Being a technically demanding procedure presenting a risk of serious injury to the patient, the implantation of intervertebral body fusion devices or partial vertebral body replacement devices should be performed only by experienced spine surgeons with specific training in the use of this system and who have knowledge of the present instruction for use.
- The surgeon should consider the location of implantation, the weight of the patient, the patient’s activity level or general conditions and any other factor which may have an impact on the performance of the system.
- Patients who smoke have been shown to have an increased risk of non-unions. Such patients should be advised of this fact and warned of the potential consequences.
- If the patient is involved in an occupation or activity which applies inordinate stress upon the implant (e.g., substantial walking, running, lifting of significant loads, or muscle strain), resultant forces can cause failure of the device.
- In some cases, progression of degenerative disease may also be so advanced at the time of implantation that they may substantially decrease the expected useful life to the device. In such cases, orthopedic devices may be considered only as a delaying technique or to provide temporary relief.
- Before clinical use, the surgeon should thoroughly understand all aspects of the surgical procedure and limitations of the system. This device is recommended for use only by surgeons familiar with preoperative and surgical techniques, cautions and potential risks associated with such spinal surgery. Knowledge of surgical techniques, proper reduction, selection and placement of implants, and pre and post-operative patient management are considerations essential to a successful surgical outcome.
- Patients should be instructed in detail about the limitations of the implants, including but not limited to the impact of excessive loading through patient weight or activity, and should be taught to govern their activities accordingly.
- Appropriate selection, placement and fixation of the spinal system components are critical factors which affect implant service life. Accordingly, strict adherence to the indications, contraindications, precautions, and warnings for this product is essential to potentially maximize service life. (Note: While proper implant selection can minimize risks, the size and shape of human bones present limitations on the size, shape, and strength of implants.)
- Supplemental internal fixation is required when using the ROI -C Implant System & ROI-C Titanium-Coated Implant System. The two piece VerteBRIDGE Anchor Plate system is available for use with the ROI-C Implant System & ROI-C Titanium-Coated Implant System, and is the supplemental fixation available for use in situations where a stand-alone construct is appropriate. The system may be augmented with additional supplemental fixation as needed and determined by the user. The instructions for use for any additional supplemental fixation system(s) should be followed according to the manufacturer’s guidelines.
- Care must be taken to protect the components from being marred, nicked or notched as a result of a contact with metal or abrasive objects. Alterations will produce defects in surface finish and internal stresses which may become the focal point for eventual breakage of the implant.
- Inspection and trial assembly are recommended prior to surgery to determine if the instruments have been damaged during storage or prior procedures.
- Sale of this product is restricted to physicians.
MRI Safety Information
Non-clinical testing has demonstrated that the Interbody Cage Systems are MR-Conditional. Patients can be scanned safely immediately after implantation under the following conditions:
- Static magnetic field of 1.5 Tesla (1.5T) or 3.0-Tesla (3.0T) only.
- Maximum spatial gradient field of 3000 G/cm (30 T/m) or less.
- Normal Operating Mode: Maximum whole-body specific absorption rate (SAR) of 3.2-3.5 W/kg for 1.5 T systems and 3.2-3.6 W/kg for 3 T systems.
- When other methods of supplemental fixation are used, also follow the MR conditional labeling for the additional components.
Under the scan conditions defined above, the ROI-C Implant System is expected to produce a maximum temperature rise of less than 1°C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 0.8 cm from the ROI-C Implant when imaged with a gradient echo pulse sequence in either a 1.5T or a 3T MRI system.
Additional Information
Contact Us
USA: 800 447-3625
To submit a complaint, please email SpineComplaints@highridgemedical.com
10225 Westmoor Dr. Westminster, CO 80021 USA
To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please call 800 447-3625.
References
- “Cadaveric Biomechanical Evaluation of the ROI-C Cervical Cage with VerteBRIDGE Plating Technology” LDR REF: IR-C T 1 EN 12.2010 B US
*In cases of trauma, vertebral instability, or significant bone removal, the ROI-C implant should be augmented with additional supplemental fixation.
Legal Manufacturer
Highridge Medical
10225 Westmoor Dr.
Westminster, CO 80021 USA

