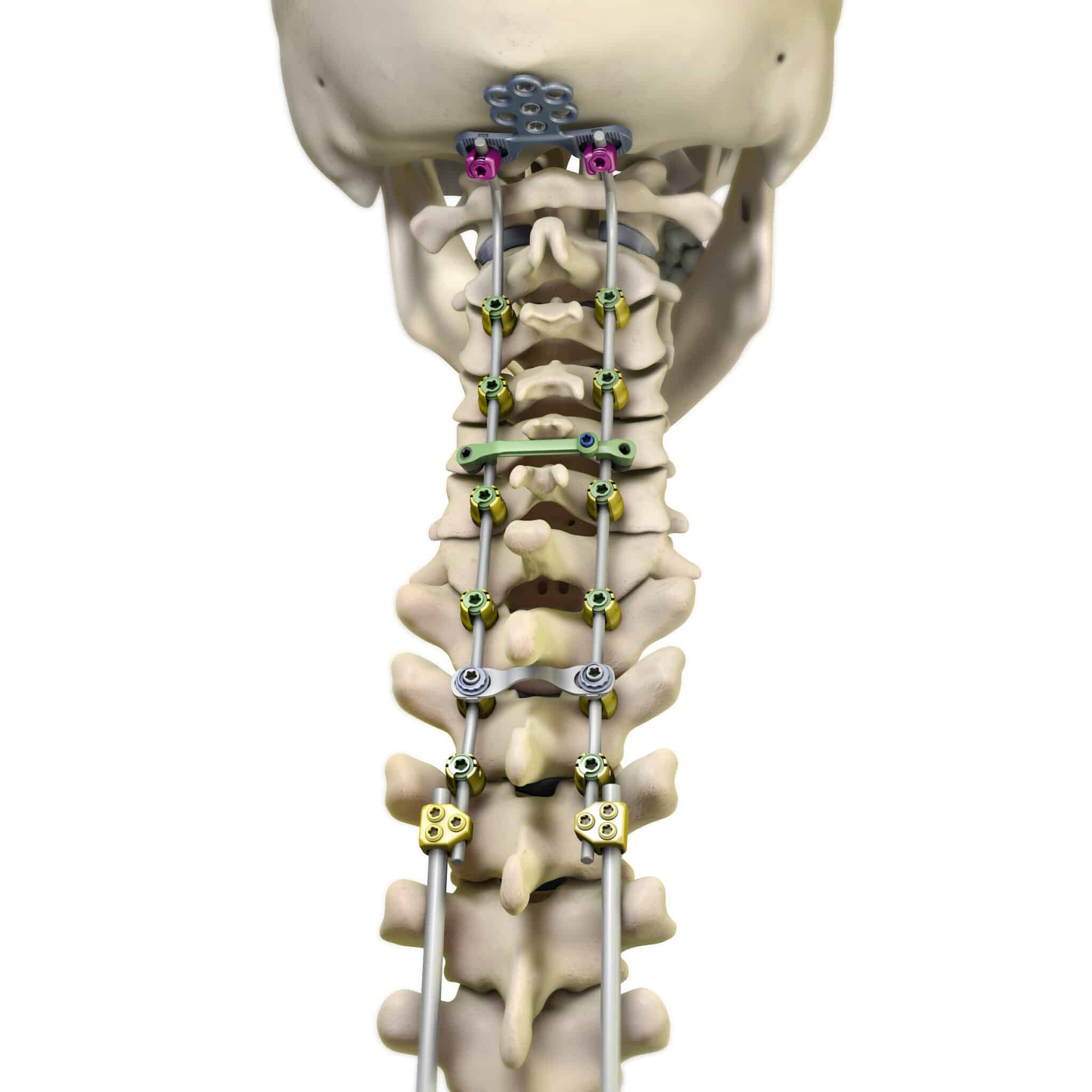
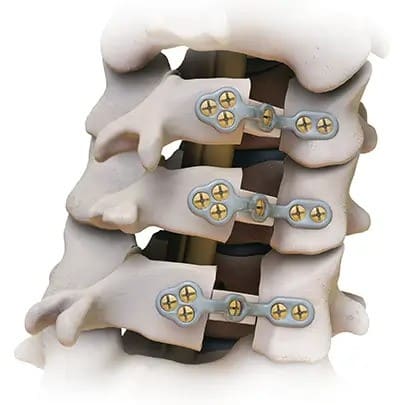
Virage® OCT Spinal Fixation System
Engineered for Performance


Movement in a New Direction
The Virage OCT System offers a new approach to posterior fixation surgery through Highridge Omnidirectional Extreme-Angle Screw. Built to deliver efficient results in the operating room, this system works to address challenging patient anatomies.
System Features
The Virage System is an Occipital-Cervico-Thoracic (OCT) spinal fixation system featuring the innovative 360° Omnidirectional Extreme-Angle Screw that simplifies rod alignment and minimizes operating time.

Flexibility
- The Virage OCT System offers the widest range of screw diameters for use in longer constructs.
- The Extreme Angle Omnidirectional screw allows for 56° of angulation and 112° of conical range of motion designed to facilitate optimal screw placement.
- Multiple rod options include Cobalt Chrome and Titanium materials, pre-cut and pre-bent rods and pre-bent and articulating occipital rods.
- Head-to-head connectors provide multi-planar motion, allowing for off-axis screw head positioning.

Efficiency
- Double-lead screw accelerates insertion.
- Omnidirectional screw simplifies rod placement and minimizes operating time.
- Friction-fit head holds the desired rod position and facilitates rod placement.

Safety
- Varying thread forms maximize screw interaction with various bone densities.
- The Virage OCT System has demonstrated to have increased pull-out strength when compared to competitive systems. (Test data on file at Highridge).
See How It Works
Dr. Rory Murphy
Dr. Chung
Dr. Lawrence
Related Products
Highridge utilizes its deep industry knowledge and expertise to refine a comprehensive anterior cervical product portfolio of next generation devices.
Resources
Brochure & Surgical Technique
Important Information
Description, Indications, and Contraindications
Description
The Highridge Medical Virage OCT Spinal Fixation System is a posterior system intended for the Occipital-Cervical-Thoracic spine (Occiput-T3). The system consists of a variety of rods, anchors, connectors, screws, and polyaxial screws to achieve an implant construct as necessary for the individual case. The system also includes the instruments necessary for inserting and securing the implants. The implant system is intended to be removed after solid fusion has occurred. The Virage System implants are fabricated from medical grade titanium alloy and medical grade cobalt chromium alloy. Implants made from medical grade titanium, medical grade titanium alloy, and medical grade cobalt chromium may be used together. Never use titanium, titanium alloy, and/or cobalt chromium with stainless steel in the same construct. All implants are single use only and should not be reused under any circumstances. Refer to the Virage OCT Spinal Fixation System Surgical Technique manual for additional information on how to use this device. Contact your Highridge Medical Sales Representative or Highridge Medical Customer Service for a copy of the current Surgical Technique.Indications
The Virage OCT Spinal Fixation System is intended to provide immobilization and stabilization of spinal segments as an adjunct to fusion for the following acute and chronic instabilities of the craniocervical junction, the cervical spine (C1-C7) and the thoracic spine from T1-T3; traumatic spinal fractures and/ or traumatic dislocations; instability of deformity; failed previous fusions (e.g.,pseudoarthrosis); tumors involving the cervical spine and degenerative disease, including intractable radiculopathy and/or myelopathy, neck and/or arm pain of discogenic origin as confirmed by radiographic studies, and degenerative disease of the facets with instability. The Virage OCT Spinal Fixation System is also intended to restore the integrity of the spinal column even in the absence of fusion for a limited time period in patients with advance stage tumors involving the cervical spine in whom life expectancy is of insufficient duration to permit achievement of fusion. In order to achieve additional levels of fixation, The Virage OCT Spinal Fixation System may be connected to the Vital Spinal Fixation System offered by Highridge Medical, using rod connectors and transition rods. Refer to the Vital Spinal Fixation System package insert for a list of the system specific indications of use.Contraindications
The Virage System is not designed or sold for any use except as indicated. DO NOT USE THE VIRAGE SYSTEM IMPLANTS INTHE PRESENCE OF ANY CONTRAINDICATION.
Contraindications include, but are not limited to:
1. Overt infection or distant foci of infections.
2. Local inflammation, with or without fever or leukocytosis.
3. Pregnancy.
4. Morbid obesity.
5. Rapid joint disease, bone absorption, osteopenia, and/or osteoporosis.
6. Suspected or documented metal allergy or intolerance.
7. Any time implant utilization would interfere with anatomical structures or expedited physiological performance, such as impinging on vital structures.
8. Severe comminuted fractures such that segments may not be maintained in satisfactory proximate reduction.
9. Use in displaced, non-reduced fractures with bone loss.
10. The presence of marked bone absorption or severe metabolic bone disease that could compromise the fixation achieved.
11. Poor prognosis for good wound healing (e.g., decubitus ulcer, end-stage diabetes, severe protein deficiency, and/or malnutrition).
12. Any case not needing a bone graft or fusion.
13. Any case not described in the indications.Warnings and Precautions
Warnings
Following are specific warnings, precautions, and adverse effects associated with use of the Virage System that should be understood by the surgeon and explained to the patients. General surgical risk should be explained to the patients prior to surgery.
- Implantation of the Virage System should be performed only by experienced spinal surgeons
- All implants are intended for single use only. Single use devices should not be re-used. Possible risks associated with re-use of single-use devices include:
- Mechanical malfunction
- Transmission of infectious agents
- INSTRUCTIONS FOR USE
- NON STERILE ONLY
- SINGLE USE
Zimmer Biomet Spine, Inc.
10225 Westmoor Dr. Westminster, CO 80021 USA
800 447-3625
highridgemedical.com Consult the Symbols Glossary at ifu.highridgemedical.com for descriptions of symbols on the device and the device labels. IFU07.01820.001-US-en Rev 08 2025-04 2- Metal sensitivity has been reported following exposure to orthopedic implants. The most common metallic sensitivities (nickel, cobalt, and chromium) are present in medical grade stainless steel and cobalt-chrome alloys.
- The Virage System is a temporary internal fixation device. Internal fixation devices are designed to stabilize the operative site during the normal healing process. After healing occurs, these devices serve no functional purpose and should be removed. Implant removal should be followed by adequate postoperative management to avoid fracture or refracture.
- Universal precautions should be observed by all end users that work with contaminated or potentially contaminated medical devices. Caution should be exercised when handling devices with sharp points or cutting edges to prevent injuries during and after surgical procedures and reprocessing.
- Warning: The safety and effectiveness of pedicle screw spinal systems have been established only for spinal conditions with significant mechanical instability or deformity requiring fusion with instrumentation. These conditions are significant mechanical instability or deformity of the thoracic, lumbar, and sacral spine secondary to severe spondylolisthesis (grades 3 and 4) of the L5-S1 vertebra, degenerative spondylolisthesis with objective evidence of neurological impairment, fracture, dislocation, scoliosis, kyphosis, spinal tumor, and failed previous fusion (pseudoarthrosis). The safety and effectiveness of these devices for any other conditions are unknown.
- Precaution: The implantation of spinal fixation systems should be performed only by experienced spinal surgeons with specific training in the use of these spinal systems because this is a technically demanding procedure presenting a risk of serious injury to the patient. Preoperative planning and patient anatomy should be considered when selecting implant diameter and length. Additional preoperative, intraoperative, and postoperative warnings and precautions:
PREOPERATIVE
PRE-OP PLANNING – Use of cross sectional imaging (i.e., CT and/or MRI) for posterior cervical screw placement is recommended due to the unique risks in the cervical spine. The use of planar radiographs alone may not provide the necessary imaging to mitigate the risk of improper screw placement. In addition, use of intraoperative imaging should be considered to guide and/or verify device placement, as necessary.
- Usage of automated cleaning processes without supplemental manual cleaning may not result in adequate cleaning of instruments.
- Proper handling, decontamination (including pre-rinsing, washing, rinsing and sterilization), storage and utilization are important for the long and useful life of all surgical instruments. Even with correct use, care and maintenance, they should not be expected to last indefinitely. This is especially true for cutting instruments (e.g., bone awls/drills) and driving instruments (e.g., drivers). These items are often subjected to high loads and/or impact forces. Under such conditions, breakage can occur, particularly when the item is corroded, damaged, nicked or scratched.
- Prior to use, instruments should be visually inspected for wear and tested to assure they are functioning properly. If instruments are discolored, show evidence of corrosion, have loose screws/pins, are out of alignment, are cracked or have other irregularities, Do Not Use. Instrumentation that appears damaged should be returned to the manufacturer.
- Never use titanium, titanium alloy, and/or cobalt chromium with stainless steel in the same implant construct; otherwise, galvanic corrosion may occur. See DESCRIPTION section for Virage System materials and compatibility information.
INTRAOPERATIVE
- If contouring of the implant is necessary for optimal fit, the contouring should be gradual and avoid any notching or scratching of the implant surface. Do not repeatedly or excessively bend the implant. Do not reverse bend the plate or rods.
- Bending Plate outside of bend zone groove may result in cracking of Plate. Surgeon should always inspect Plate before implanting.
- Occiput and pedicle bone integrity should be verified
- Care should be taken during occiput and pedicle preparation to avoid penetrating too deep.
- Care should be taken to ensure occipital screw is not driven in too deep
- Care should be taken during bone preparation to avoid damage to the pedicle and to the surgical instruments.
- Care should be taken to minimize soft tissue damage during surgery.
- Care should be taken to avoid removing excess material from the Lamina.
- Care should be taken to avoid cross-threading screws and closure tops.
- If any implant or instrument comes in contact with a nonsterile surface it should not be used.
POSTOPERATIVE
- Adequately instruct the patient. Postoperative care and the patient’s ability and willingness to follow instructions are one of the most important aspects of successful bone healing. The patient must be made aware of the limitations of the implant and that physical activity and full weight bearing have been implicated in fracture. The patient should understand that an implant is not as strong as normal, healthy bone and will fracture if excessive demands are placed on it in the absence of complete bone healing. An active, debilitated, or demented patient who cannot properly use weight-supporting devices may be particularly at risk during postoperative rehabilitation.
- The Virage System is a temporary internal fixation device.Internal fixation devices are designed to stabilize the operative site during the normal healing process. After healing occurs, these devices serve no functional purpose and should be removed. Implant removal should be followed by adequate postoperative management to avoid fracture or refracture.
- Metal sensitivity has been reported following exposure to orthopedic implants. The most common metallic sensitivities (nickel, cobalt, and chromium) are present in medical grade stainless steel and cobalt-chrome alloys.
- The Virage System is a temporary internal fixation device. Internal fixation devices are designed to stabilize the operative site during the normal healing process. After healing occurs, these devices serve no functional purpose and should be removed. Implant removal should be followed by adequate postoperative management to avoid fracture or refracture.
Additional Information
Contact Us
USA: 800 447-3625
To submit a complaint, please email spinecomplaints@highridgemedical.com
10225 Westmoor Dr. Westminster, CO 80021 USA
To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please call 800 447-3625.
Legal Manufacturer
Zimmer Biomet Spine, Inc.
10225 Westmoor Dr.
Westminster, CO 80021 USA