Universal Navigation System
The HIGHRIDGE Universal Navigation System was designed for compatibility with the Medtronic® StealthStation® System and the SureTrak™ II clamps and arrays.
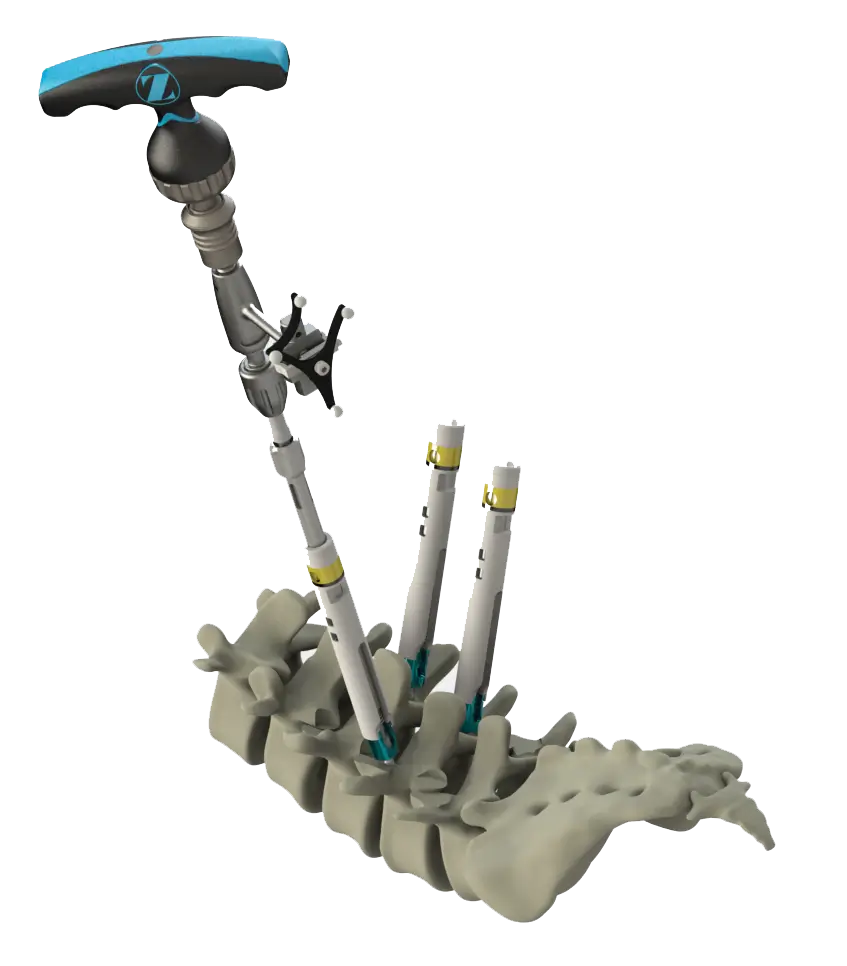

Ergonomic Design
The HIGHRIDGE Universal Navigation Adaptor is ergonomically designed to facilitate rotation of the arrays relative to the instrument axis for uninterrupted navigation.
System Features
The following HIGHRIDGE systems may be utilized with the Universal Navigation System: Vitality® Spinal Fixation System, Vital™ MIS Spinal Fixation System, PathFinder® NXT Minimally Invasive Pedicle Screw System, Cypher™ MIS Screw System and the Polaris™ Spinal Fixation System.
Suitable for a MIS or Open Approach
- Uninterrupted navigation facilitated by a freely-rotating adaptor
- Ergonomically designed to facilitate the hand-held stabilization of the array relative to the instrument
Compatibility
- Compatible with multiple navigation-ready systems (Medtronic StealthStation Navigation System and ROSA ONE)
- The surgical instruments to be assembled and navigated using the Universal Adapter are:
- PathFinder NXT taps and screwdrivers
- Cypher taps and screwdrivers
- Vitality/Vital taps and screwdrivers
- Polaris taps, reamer probe and screwdrivers
- Vital Power Reamer Probes
- Universal Nav PAT/PASIT for use with Vital MIS Spinal Fixation
Resources
Surgical Technique
Important Information
Description and Indications
Description
When used with the ROSA ONE System, the ZimVie Universal Navigation System includes Universal Adapters that are intended to be used with instrumentation from Vital™ MIS Screw System, PathFinder NXT® Minimally Invasive Pedicle Screw System, and Cypher™ MIS Screw System to facilitate preparation and insertion of ZimVie screws using navigation.
When used with the Medtronic StealthStation System, the ZimVie Universal Navigation System includes Universal Adapters that are intended to be used with instrumentation from Vitality Spinal Fixation System (including Vital™ Power Instrument System), PathFinder NXT Minimally Invasive Pedicle Screw System, Cypher MIS Screw System and Polaris™ Spinal System to facilitate preparation and insertion of ZimVie screws using navigation.
As with all orthopedic surgical procedures, detailed preoperative planning is essential. Preoperative diagnostic evaluation, followed by carefully executed surgical technique is required. Postoperative care, individualized to suit the particular injury/disease requirements, is essential for optimum outcome. The surgeon must be fully aware of the risks and complications inherent to this type of surgery. Only those individuals with specialized training and experience in spinal surgery should attempt use of the instruments.
The instrument cases may be multi-layered with various inserts to hold surgical instrumentation in place during handling and storage. The inserts may consist of trays and holders. The instrument cases are perforated to allow steam to penetrate these various materials and components. The instrument cases will allow for sterilization of the contents to occur in a steam sterilizer utilizing a cycle that has been validated by the user for the equipment and procedures employed at the user facility. Instrument cases do not provide a sterile barrier and must be used in conjunction with a sterilization wrap or rigid container to maintain sterility.
Indications
When used with the ROSA ONE System, the ZimVie Universal Navigation System is indicated for use during the preparation and insertion of ZimVie screws during spinal surgery to assist the surgeon in precisely locating anatomical structures in either open or minimally invasive procedures. The universal adaptors are specifically designed for use with the ZimVie ROSA ONE System, which is indicated for that the required fiducial markers and rigid patient anatomy can be identified on 3D CT scans. The ROSA ONE Spine System is intended for the placement of pedicle screws in vertebrae with a posterior approach in the thoracolumbar region.
Indications for compatible systems, Vital MIS Screw System, PathFinder NXT Minimally Invasive Pedicle Screw System, and the Cypher MIS Screw System can be found in their respective system IFUs.
When used with the Medtronic StealthStation System, the ZimVie Universal Navigation System is indicated for use during the preparation and insertion of ZimVie screws during spinal surgery to assist the surgeon in precisely locating anatomical structures in either open or minimally invasive procedures. The universal adapters are specifically designed for use with the Medtronic StealthStation System which is indicated for any medical condition in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure, such as a pelvis or vertebra can be identified relative to an acquired image: CT or MR based model, fluoroscopy images, or digitized landmarks of the anatomy.
Indications for compatible systems, Vitality Spinal Fixation System (including Vital Power Instrument System), PathFinder NXT Minimally Invasive Pedicle Screw System, Cypher MIS Screw System and Polaris Spinal System, can be found in their respective system IFUs.
Warnings and Precautions
Following are specific warnings, precautions, and adverse effects associated with use of the Universal Adapters for Navigation non-sterile instrumentation that should be understood by the surgeon and explained to the patients. General surgical risk should be explained to the patients prior to surgery.
Warnings
- For use with the ROSA ONE system it is important not to exceed an instrument length of 360 mm for awls, taps and screwdriver/screw assemblies. The use of longer instrumentation could lead to a decrease in system accuracy.
- For use with the Medtronic StealthStation system, it is important not to exceed the instrument lengths stated for each array in Table 1. If the arrays are used outside of the scope of Table 1 it could lead to decreased accuracy for the system.
- When tightening the collet nut to rigidly affix the instrument into the adaptor shaft, the assembly should be held in a vertical position and the collet nut key should be held perpendicular to the shaft. Neglecting to assemble this instrument in this way could lead to decreased accuracy for the system.
- For use with the ROSA ONE system, when adding a new instrument to an already calibrated array, the calibration step must be re-performed. Failure to re-calibrate after changes to the instrument or instrument adapter assembly can lead to decreased accuracy for the assembly.
- For use with the Medtronic StealthStation system, when adding a new instrument to an already calibrated array or a new implant to an instrument/implant assembly which has already been calibrated, the calibration step must be re-performed. Failure to re-calibrate after changes to the instrument or instrument/implant assembly can lead to decreased accuracy for the assembly.
- Breakage, slippage, misuse, or mishandling of instruments, such as on sharp edges, may cause injury to the patient or operative personnel.
- Improper maintenance, handling, or poor cleaning procedures can render the instrument unsuitable for its intended purpose or even dangerous to the patient or surgical staff.
- The physical characteristics required for many instruments does not permit them to be manufactured from implantable materials, and if any broken fragments of instruments remain in the body of a patient, the patient could have allergic or infectious consequences.
- It is important that the surgeon exercise extreme caution when working in close proximity to vital organs, nerves or vessels, and that the forces applied while correcting the position of the instrumentation is not excessive, such that it might cause injury to the patient.
- ZimVie does not warrant Medtronic Navigation Software. It is the sole responsibility of the user to ensure instrument calibration and/or registration.
- Users must complete instrument registration steps as required per the Medtronic Navigation Operative Technique.
- Users must ensure that surgical accuracy be assessed before the procedure and repeatedly throughout the procedure by positioning the tip of each navigated instrument on an identifiable anatomical landmark and comparing the actual tip location to that displayed by the system. When verifying the accuracy of the Navigated drivers, the accuracy test must include the screw assembled securely onto the driver. The screw tip will be placed on an identifiable anatomical landmark and compared to the tip location as displayed on the screen.
- In the event of a registration failure or suspected inaccuracy, Navigation should be discontinued and the instruments should be inspected for damage before continuing with the traditional, non-navigated procedure.
Precautions
- A successful result is not always achieved in every surgical case. This fact is especially true in spinal surgery, where many extenuating circumstances may compromise the results.
- Proper patient selection and operative care are critical to the success of the device and avoidance of injury during surgery.
- Read and follow the instructions for use and surgical technique guide provided by the manufacturer for this product.
- Under no circumstances are these instruments to be implanted.
Preoperative Precautions
- Usage of automated cleaning processes without supplemental manual cleaning may not result in adequate cleaning of instruments.
- Proper handling, decontamination (including pre-rinsing, washing, rinsing and sterilization), storage and utilization are important for the long and useful life of all surgical instruments. Even with correct use, care and maintenance, they should not be expected to last indefinitely. This is especially true for cutting instruments (e.g., bone awls/drills) and driving instruments (e.g., drivers). These items are often subjected to high loads and/or impact forces. Under such conditions, breakage can occur, particularly when the item is corroded, damaged, nicked or scratched.
- Zimmer Biomet does not specify the maximum number of times a re-usable instrument may be re-used. The useful life of these instruments is highly dependent on a number of factors including the frequency and manner in which they are used and the handling they experience in between uses.
- Prior to use, instruments should be visually inspected for wear and tested to assure they are functioning properly. If instruments are discolored, show evidence of corrosion, have loose screws/pins, are out of alignment, are cracked or have other irregularities, DO NOT USE. Instrumentation that appears damaged should be returned to the manufacturer.
Intraoperative Precautions
- Over-bending, notching, striking and scratching with any instruments should be avoided to reduce the risk of breakage.
- If any instrument comes in contact with a non-sterile surface it should not be used.
- Extreme care must be taken when used near vital organs, nerves or vessels.
Possible Complications
- Nerve damage, paralysis, pain, or damage to soft tissue, visceral organs or joints.
- Infection, if instruments are not properly cleaned and sterilized.
- Nerve damage due to surgical trauma.
- Impingement of close vessels, nerves, and organs by slippage or misplacement of the instrument.
- Cutting of skin or gloves of operating staff.
- Bony fracture in cases of deformed spine or weak bone.
- Involuntary crack, fracture or perforation of the bone.
- The methods of use of instruments are to be determined by the user’s experience and training in surgical procedures.
Additional Information
Contact Us
USA: 800 447-3625
To submit a complaint, please email SpineComplaints@zimvie.com
10225 Westmoor Dr. Westminster, CO 80021 USA
To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please call 800 447-3625.
Legal Manufacturer
Zimmer Biomet Spine, Inc.
10225 Westmoor Dr.
Westminster, CO 80021 USA