Highridge offers an All-in-One Tether Certification Course that consists of a faculty led didactic presentation on VBT (Vertebral Body Tethering), hands-on training focused on the The Tether™ Instrumentation and surgical workflow, a physician dinner to facilitate case discussion, and an O.R. observation visit.
The Tether™ Vertebral Body Tethering System
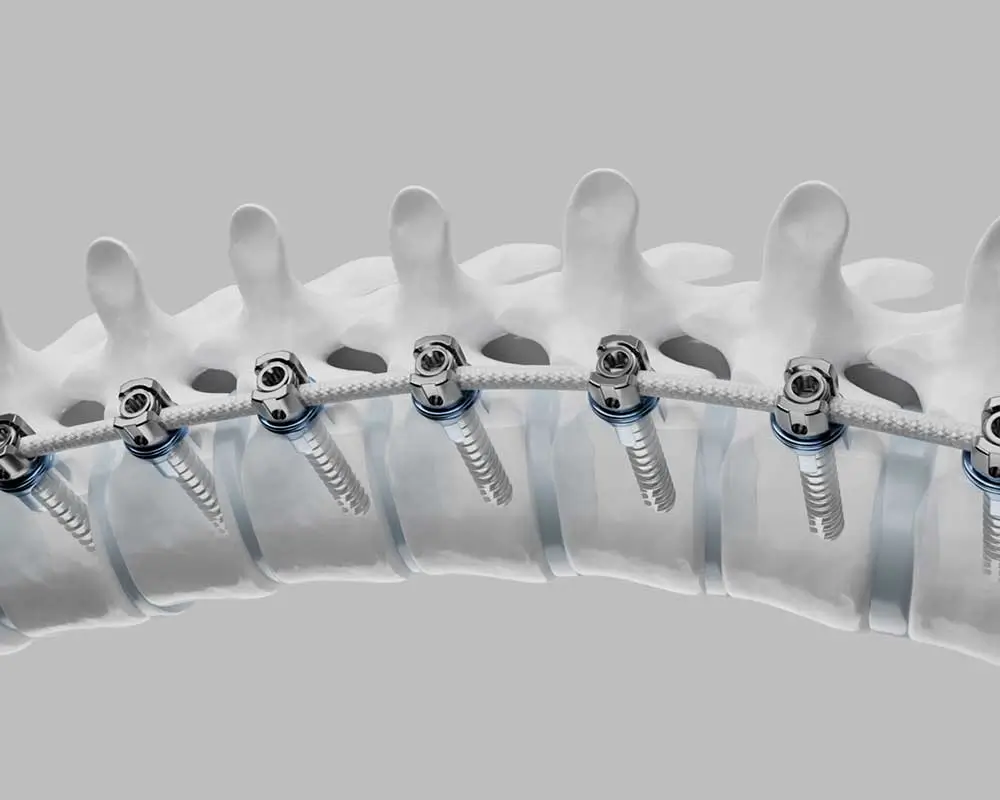
The Tether™ is the first and FDA approved vertebral body tethering system. A revolutionary new device designed for correction of adolescent scoliosis, while preserving motion of the spine.

Overview
Ahead Of The CurveTM
The Tether™ – Vertebral Body Tethering System is a non-fusion spinal device intended for treatment of idiopathic scoliosis. Anchors and vertebral body screws are placed laterally from a thoracoscopic or thoracotomy approach into the vertebral body on the convex side of a spinal deformity.
A SULENE® polyethylene terephthalate (PET) tensioning cord is secured to the vertebral body screws with set screws to connect the levels of the construct. The device provides a lateral tension band across the convex side of the spine that, on insertion and tensioning, partially corrects the curvature, and subsequently can arrest or correct the deformity through modulation of remaining spinal growth. In addition, the subject system includes instrumentation for insertion, manipulation, and removal of the implants.
Clinical Results
The purpose of this study was to assess the safety and probable benefit of the device in subjects with idiopathic scoliosis. Spinal tethering subjects were retrospectively evaluated for clinical and radiographic outcomes and were then prospectively followed until 30 out of 57 (47.4%) reached skeletal maturity by the time of database lock.
Clinical Data Overview
Zimmer Biomet Spine conducted a single-center, non-randomized, clinical study under Investigational Device Exemption (IDE) application G150001 in fifty-seven (57) subjects. The purpose of this study was to assess the safety and probable benefit of the device in subjects with idiopathic scoliosis. Spinal tethering subjects were retrospectively evaluated for clinical and radiographic outcomes and were then prospectively followed until 30 out of 57 (47.4%) reached skeletal maturity by the time of database lock. All subjects were surgically treated utilizing components of the Dynesys® Top-Loading Spinal System which is cleared for spinal fusion (K133164). The Tether™ - Vertebral Body Tethering System includes similar components, but differs from the Dynesys® System in that screws have a lower profile head. A common primary assessment collected for all subjects was curve magnitude as determined by Cobb angle. Radiographic images were analyzed using a single core laboratory for assessment of coronal Cobb angle, device loosening, and device breakage. AEs were also reported and assessed by each investigator.
Non-Clinical Data
A number of non-clinical tests were conducted on The Tether™ - Vertebral Body Tethering System, including static and dynamic tension bending, static axial grip, creep, stress relaxation and wear testing. All tests passed pre-determined acceptance criteria.
System Features
The Tether™ - Vertebral Body Tethering System™ is different from the other surgical treatments for scoliosis — e.g. spinal fusion — because the spine is still able to bend and flex, rather than being fixed in place with the stiff metal rods needed for spinal fusion.

There may be smaller incisions required (less scarring).

Designed to allow for motion at the levels treated.

There may be less muscle and soft tissue disruption.

Specifications
Materials
The Tether™ – Vertebral Body Tethering System is manufactured from:
- Anchors: Ti 6Al-4V ELI titanium alloy per ASTM F136
- Set Screws: Ti 6Al-4V ELI titanium alloy per ASTM F136
- Bone Screws: Ti 6Al-7Nb titanium alloy per ISO 5832-11 with an hydroxyapatite coating per ISO 13779-2
- Cord: Sulene® PET (polyethylene-terephthalate)
- Instruments and Cases: Generally comprised of aluminum, stainless steel, titanium alloy, and/or polymeric materials
Vertebral Body Anchors
Vertebral Body Screw:
12 mm Vertebral Body Anchor.
The vertebral body screw for the proposed Zimmer Biomet Spine’s The Tether, is a modified version of the current Dynesys Top-Loading Spinal System’s pedicle screw.
Anchor:
It should be noted that in previous submissions concerning The Tether, this component was referred to as a “staple”. This device will be referred to as an anchor in this submission and any labeling to avoid confusion with devices used for vertebral body stapling procedures.
The anchor is optionally used to provide additional resistance to migration of the screw when implanted in the vertebral body. After preparation for and placement of the anchor in the vertebral body, the screw is placed through the center of the anchor.
The Dynesys system does not include an anchor as it is not indicated for tethering procedures. Anchors available on the market have been used for tethering procedures to help prevent screw migration. The Tether’s anchor has been designed to interface with the vertebral body screw to similarly achieve this purpose.
Vertebral Body Bone Screws
Set Screw:
The Tether set screw is a modified version of the current Zimmer Dynesys Top- Loading System set screw. The tip has been slightly modified to simplify the cord contact area with the set screw.
After screw placement and cord tensioning, the set screws are used to secure the cord to the screw and maintain tension. The set screws are tightened to 50 in-lbs to ensure there is proper engagement with the cord. Under-tightening may result in cord slippage while over-tightening may damage the tensioning cord and lead to cord breakage.
Flexible PET Cord
Tensioning Cord:
The Tether Tensioning Cord is similar to the cords available for the Dynesys Top-Loading System, but it only comes in one longer length to better accommodate the new intended use and patient population (300 mm). This allows for sufficient cord length when creating a tethering construct and the surgeon is able to trim any additional cord length intra-operatively.
The introduction zone is stiffer than the rest of the cord due to a solid core. This allows for it to be more easily threaded through the tulips of the vertebral body screws. The working zone is identified by blue striations and is simply a result of the manufacturing process to transition from the non-braided introduction zone to the functional zone in one continuous cord; the working zone better allows for the manufacturing process to achieve a more consistent product through the functional zone. Finally, the functional zone is the portion of the cord used to create tension between the levels of the spine and ultimately be implanted. The working and introduction zones are always severed from the functional zone after final tensioning as they no longer serve a functional purpose.
HIGHRIDGE Institute
Global Education Event Calendar
Register for any of the following programs to experience what the Company has to offer!
Spine Educational Calendar
Resources
Surgical Technique
For more information and to register, please reach out to your HIGHRIDGE Sales Representative.
If you are interested in training certification on The Tether™:
Training Course
Surgeon-to-Surgeon Visitation Program
Peer-to-Peer Discussion
Educational Site Visit
If you are interested in increasing local awareness:
Surgeon-to-Surgeon Visitation Program
Peer-to-Peer Discussion
Important Information
The Tether™ is the first and FDA approved vertebral body tethering system. A revolutionary new device designed for correction of adolescent scoliosis, while preserving motion of the spine.
Humanitarian Device
Humanitarian Device. Authorized by Federal law for use in the treatment of skeletally immature patients that require surgical treatment to obtain and maintain correction of progressive idiopathic scoliosis, with a major Cobb angle of 30 to 65 degrees whose osseous structure is dimensionally adequate to accommodate screw fixation, as determined by radiographic imaging. Patients should have failed bracing and/or be intolerant to brace wear. The effectiveness of this device for this use has not been demonstrated.
Post-operative risks include overcorrection of the instrumented curve, cord breakage and bone screw migration. Full contraindication and risk information can be found at myscoliosis.com
References
- Cote P et al. “A study of the diagnostic accuracy and reliability of the Scoliometer and Adam’s forward bend test.” Spine. 1998: 23(7), 796-802.
- Risser JC. “The iliac apophysis: an invaluable sign in the management of scoliosis.” Clinical Orthopaedics. 1958: 11,111- 119.
- Sanders JO et al.. “Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence.” The Journal of Bone and Joint Surgery. 2008: 90(3), 540-553.
Unless otherwise indicated, as referenced herein, all trademarks and intellectual property rights are the property of Highridge Inc. or an affiliate; and all products are manufactured by one or more of the spinal subsidiaries of Highridge Inc. (Zimmer Biomet Spine, Inc., Zimmer Spine, LDR Medical, etc.) and marketed and distributed by Highridge Spine and its authorized marketing partners. The Tether is manufactured by Highridge Spine, Inc. For additional product information, please refer to the individual product labeling or instructions for use. Product clearance and availability may be limited to certain countries/regions. This material is intended for clinicians only and does not comprise medical advice or recommendations. Distribution to any other recipient is prohibited. This material may not be copied or reprinted without the express written consent of Highridge. ZV0301 REV B 05/23 ©2023 Highridge Inc. All rights reserved.
Indications and Contraindications
Indications
The Tether™ – Vertebral Body Tethering System is indicated for skeletally immature patients that require surgical treatment to obtain and maintain correction of progressive idiopathic scoliosis, with a major Cobb angle of 30 to 65 degrees whose osseous structure is dimensionally adequate to accommodate screw fixation, as determined by radiographic imaging. Patients should have failed bracing and/or be intolerant to brace wear.
Contraindications
The Tether™ – Vertebral Body Tethering System should not be implanted in patients with the following conditions:
- Presence of any systemic infection, local infection, or skin compromise at the surgical site;
- Prior spinal surgery at the level(s) to be treated;
- Known poor bone quality defined as a T-score -1.5 or less;
- Skeletal maturity;
- Any other medical or surgical condition which would preclude the potential benefit of spinal surgery, such as coagulation disorders, allergies to the implant materials, and patients unwillingness or inability to cooperate with post-operative care instructions.
Pre-Operative and Operative Procedure
Preoperative Procedure
The surgeon is responsible for being familiar with the indications, contraindications, system/procedure risks, and surgical technique to ensure proper treatment, patient selection, and postoperative care when using The Tether™ – Vertebral Body Tethering System. The patient must be an acceptable surgical risk, and appropriate for vertebral body tethering based on consideration of various factors such as preoperative Cobb angle, curve flexibility, curve type(s), curve location(s), skeletal maturity, and anticipated growth, among others. Examination and evaluation of the individual patient anatomy is necessary to plan the appropriate surgical procedure and technique. Due to smaller vertebral body size and variable venous anatomy, caution should be observed if extending instrumentation proximal to T5.
Zimmer Biomet Spine does not specify the maximum number of times a re-usable instrument may be re-used. The useful life of these instruments is highly dependent on a number of factors including the frequency and manner in which they are used and the handling they experience in between uses. Inspection and, where appropriate, functional testing prior to instrument use is the best way to determine whether or not an individual device should be used. Review and inspect all instrumentation and implants prior to use. Replace or add any needed components for the planned surgery.
Use of automated cleaning processes without supplemental manual cleaning may not result in adequate cleaning of instruments. Proper handling, decontamination (including
pre-rinsing, washing, rinsing and sterilization), storage and utilization are important for the long and useful life of all surgical instruments. Even with correct use, care and maintenance, instruments should not be expected to last indefinitely. This is especially true for cutting instruments (e.g., bone awls/drills) and driving instruments (e.g., drivers). These items are often subjected to high loads and/or impact forces. Under such conditions, breakage can occur, particularly when the item is corroded, damaged, nicked or scratched.
Operative Procedure
For surgical placement of The Tether™ – Vertebral Body Tethering System, patients are positioned in the lateral decubitus position with the convex side of the curve to be instrumented facing upwards. As most idiopathic thoracic curves are convex towards the right side, a left lateral decubitus position will be the most common position utilized for instrumentation of thoracic curves. For recommended surgical site preparation, positioning, and technique details, please see the Surgical Technique Guide. For thoracoscopic surgery, standard anesthesia protocol should be observed. However, it is recommended to use a single lung ventilation technique such as a double-lumen endotracheal tube to aid surgical exposure if necessary. Anchor use is recommended at all levels. Consideration should be given to the osseous structure at each level to determine if both a bone screw and anchor are needed to adequately support the construct and anticipated loads. Please refer to the Surgical Technique Guide if implant removal is required (including revision). Close wound(s) and apply wound dressing using standard techniques.
Post-Operative Care
It is critical that patients follow all postoperative instructions provided by care providers including recommendations regarding medications, home care, surgical wound dressings and activity limitations. The patient must be warned to avoid falls or sudden jolts. Failure to follow postoperative instructions could lead to impaired wound healing, injury to neurologic structures or failure to achieve desired curve correction.
Optional protective bracing may be used for up to six months after surgery depending on patient factors such as bone quality and activity levels. Bracing may provide additional support for patients who are overcorrecting or have a suspected or confirmed cord break. Use of The Tether does not preclude the need to brace compensatory curves.
Warnings & Precautions
Warnings
- Do not rotate the screw counterclockwise after insertion. This will reduce stability of the implant in the vertebral body.
- New or increasing coronal angulation between consecutive screws as seen on radiographs may indicate tether breakage. Tether breakage may be associated with loss of correction, overcorrection or may have no clinical significance.
- Never use titanium alloy(s) with stainless steel in the same implant construct; otherwise, galvanic corrosion may occur.
- When tensioning the construct, special consideration should be given to patients with considerable growth remaining (e.g. open triradiate cartilage), lower magnitude Cobb angles, and/or high flexibility as excessive tension may increase the risk of overcorrection.
- The Tether™ – Vertebral Body Tethering System must not be used with vertebral components or instruments from other manufacturers. Specialized instruments are designed for Zimmer Biomet Spine implant systems to aid in proper implantation. The use of instruments or implant components from other systems can result in inaccurate fit, incorrect sizing, excessive wear and device failure.
- Surgical instruments are subject to wear with normal usage. Instruments with cutting functions or points may become dull with normal use and no longer perform as intended. Instruments that have experienced extensive use are susceptible to fracture.
- Do not modify instruments. Do not notch, bend, or reshape instruments. Notches, scratches or other damage and/or wear in the instrument occurring during surgery may contribute to breakage. Do not use an instrument that has become bent from its original shape as this will affect the performance of the instrument. Bent instruments should be disposed of by a Zimmer Biomet Spine representative or according to hospital procedures.
- The use of an instrument for tasks other than those for which they are indicated may result in damaged or broken instruments.
- Proper handling of the instruments and implants before and during the operation is crucial. Do not apply excessive force; misuse can damage instruments or implants.
Precautions
- Due to smaller vertebral body size and variable vascular anatomy, caution should be observed if extending instrumentation proximal to T5.
- Care should be taken when using instruments over a guidewire to avoid unintentional advancement of the guidewire which could result in damage to vessels, spinal cord, or lungs.
- Extreme caution must be taken to avoid damage to vessels, spinal cord, or lungs during placement of instruments and implants.
- The Tether™ – Vertebral Body Tethering System is intended to be used by surgeons specialized in spinal surgery with thorough knowledge of vertebral anatomy, regional vertebral morphology, and biomechanical principles of the spine. The surgical procedure is technically demanding and presents a risk of serious injury to the patient. It is advised that the surgeon also be thoroughly familiar with the surgical techniques, equipment, and instruments related to the use of the device. The surgical technique guide may be obtained by contacting Zimmer Biomet Spine Customer Service (contact information is provided below).
- Risks associated with spine surgery, neurosurgery, general surgery, and the use of general anesthesia should be explained to the patient prior to surgery. It is recommended that the advantages and disadvantages of using The Tether™ – Vertebral Body Tethering System, as well as alternative treatment methods, are explained to the patient.
- The cord implant is made up of three-segments: The Introduction Zone, the Working Zone, and the Functional Zone. Only the Functional Zone may be implanted into the patient.
- Correct selection and placement of the implants is critical. Implant selection must be based upon the levels to be treated as well as the patient’s weight and height.
- Perform a careful preoperative review to be sure that all necessary implant components are available and that the instrument set is complete and in working order prior to initiating surgery.
- Before use, inspect all instrumentation for possible damage, wear, or non-function. Damaged or defective instruments should not be used or processed. Contact your local Zimmer Biomet Spine representative or distributor for repair or replacement.
- Do not reuse single-use devices such as implants. While a single-use device may appear undamaged, previous stress may have created imperfections that would reduce the service life of the single-use device. Do not treat patients with single-use devices that have been in contact with a different patient.
- All trial, packaging, and instrument components must be removed prior to closing the surgical site.
- Unless otherwise indicated, instruments are supplied NON-STERILE and must be thoroughly cleaned and sterilized prior to use. Instruments that are not clean may not be effectively sterilized.
- Automated cleaning using a washer/disinfector alone may not be effective for complex orthopaedic instruments with lumens, cannulations, blind holes, mated surfaces and other features.
- Do not clean soiled instruments while in polymer or metal trays.
Additional Information
To request a paper copy of the Instructions for Use, contact Highridge Medical Customer Service.