Mobi-C® Cervical Disc
The leading choice for cervical motion preservation


Overview
Company Announces Over 225,000 Cervical Discs Have Been Replaced with Mobi-C.
Mobi-C Cervical Disc was the first cervical disc in the United States approved to treat more than one level of the cervical spine. Mobi-C was determined by the FDA to be statistically superior to fusion at 7 years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial. At 10 years, all patient-reported outcomes were equivalent to or improved from 7 years.
Proven Clincial Success
MOBI-C Clinical Trial Results
Mobi-C was first implanted in Europe in November 2004.
To receive approval in the United States, an Investigational Device Exemption (IDE) clinical trial was conducted involving 599 patients that began in April 2006. This study compared Mobi-C to the current standard of care, anterior cervical discectomy and fusion (ACDF), for both one and two-level indications.

Ten-Year Outcomes of 1- & 2-Level Mobi-C Patients
Extended Follow-Up from the Mobi-C IDE Study
Download Clinical Trial PDFFeatures & Benefits
Mobile Bearing Technology
Restoring natural motion to the cervical spine
The controlled mobility of the patented mobile core is the foundation of Mobi-C. With vertebrae and neck muscle movement, the Mobi-C implant is free to twist and slide left-to-right, and front-to-back, as well as rotate.


Mobi-C is composed of three parts: two metal plates and a medical grade polyethylene insert in between. The top plate rotates over the domed insert, allowing for a continuous path of cyclic movements: Flexion-Extension (FE), Lateral Bending (LB), and Axial Rotation (AR)


Self-Adjusting
A return to physiological mobility
The center of rotation at each level of the cervical spine is variable and constantly changing. Mobi-C was designed to adapt to the Instantaneous Axis of Rotation through its self-adjusting mobile core. The mobile core allows the vertebrae above and below the disc to move, to maintain normal neck motion.
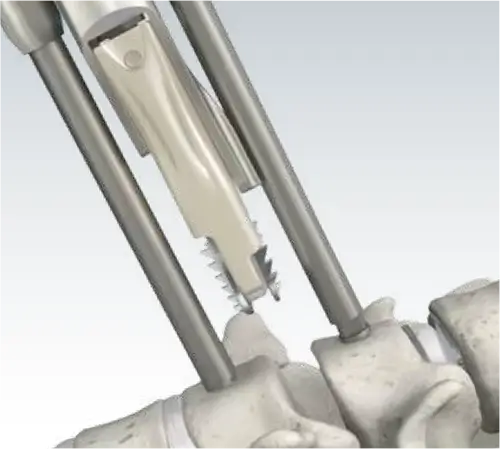
Ease of Insertion
One-Step Insertion
To insert the Mobi-C Cervical Disc, no additional exposure or operative steps are required for screw or keel placement, eliminating the need for drilling or chiseling.

The PEEK Cartridge allows a radiolucent view of the implant for optimal positioning.
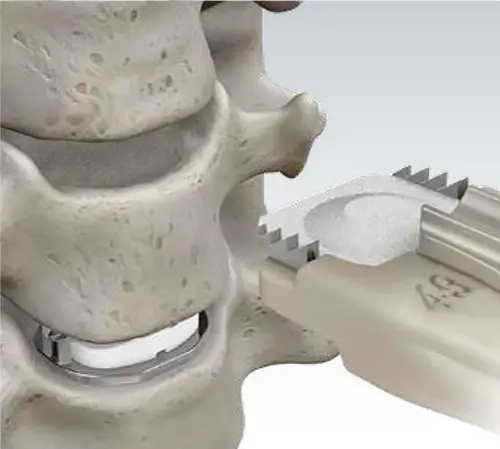
Pre-Assembled Implants
Mobi-C is delivered pre-assembled on a disposable PEEK cartridge. The cartridge assembles easily to the implant inserter, saving operative steps.

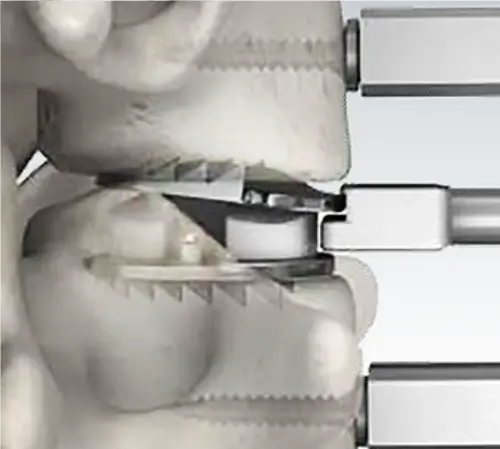
Bone Sparing
Preserves Bone Surface
Mobi-C’s mobile core is designed to create low stress at the implant to bone interface. Implantation of the Mobi-C requires no invasive keels or screws, no bone removal for keel preparation, and no additional operative steps for keel cutting.
Intact endplates, compared to endplates prepared for keels, provide a couple of benefits:
- Preserved surface for the implant, ideal for two-level implantation
- Intraoperative flexibility to optimize implant positioning

HIGHRIDGE Institute
Global Education Event Calendar
Register for any of the following programs to experience what the Company has to offer!
Spine Educational Calendar
Resources
Clinical Summaries
Important Information
Mobi-C is the first cervical disc FDA approved for both one and two-level indications. The U.S. Food and Drug Administration (FDA) approved an update to the Mobi-C labeling to include seven-year clinical results.
References
1. Data on file. Based on available market data at the time of this publication.
2. Kim K, Hoffman G, Bae H, et al. Ten-Year Outcomes of 1- and 2-Level Cervical Disc Arthroplasty From the Mobi-C Investigational Device ExemptionClinical Trial. Neurosurgery. 2021;88(3):497-505.
3. Radcliff K, Davis RJ, Hisey MS, et al. Long-term evaluation of cervical disc arthroplasty with the Mobi-C Cervical Disc: a randomized, prospective, multicenter clinical trial with seven-year follow-up. Int J Spine Surg 2017;11(4):244-262.
4. Amevo B, et al Instantaneous axes of rotation of the typical cervical motion segments: a study in normal volunteers. Clin Biomech (Bristol, Avon). 1991 May:6(2):111-7
Disclaimer
Common post-operative risks from surgery with the Mobi-C include pain in the neck, arm, back, shoulder, or head, and dysphagia.
This document is intended exclusively for physicians and is not intended for laypersons. Information on the products and procedures contained in this document is of a general nature and does not represent and does not constitute medical advice or recommendations. Because this information does not purport to constitute any diagnostic or therapeutic statement with regard to any individual medical case, each patient must be examined and advised individually, and this document does not replace the need for such examination and/or advice in whole or in part.
The clinical data presented is from use of the Mobi-C US implant design which has minor design differences compared to the Mobi-C in other countries.
Unless otherwise indicated, all trademarks are the property of Highridge. For additional product information, such as risks and contraindications, please refer to the individual product labeling or instructions for use. Product clearance and availability may be limited to certain countries/regions. This material is intended for clinicians only and does not comprise medical advice or recommendations. This material may not be copied or reprinted without the express written consent of Highridge.
©2025 Highridge Medical, LLC. All rights reserved.
Description, Indications, and Contraindications
The Mobi-C® Cervical Disc Prosthesis (Mobi-C®) is a single use device for cervical intervertebral disc replacement at one level or two contiguous levels from C3 to C7 designed to maintain/restore segmental motion and disc height. The components of the Mobi-C® include a cobalt, chromium, molybdenum (CoCrMo per ISO 5832-12) alloy superior spinal plate, an inferior CoCrMo spinal plate, and an ultra-high molecular weight polyethylene (UHMWPE per ISO 5834-2) mobile insert. The inner contact surfaces of the superior and inferior spinal plates are spherical and flat, respectively. This allows for fully congruent contact surfaces between the spinal plates and mobile insert. The two lateral stops of the inferior plate are designed to control and limit the mobility of the mobile insert. The spinal plates, both superior and inferior, feature two rows of teeth which are designed to aid in initial and long term fixation and stability. The teeth are designed to sink into the bone to facilitate endplate fixation and do not require any bone removal or chiseling prior to insertion. A titanium (per ASTM F1580) and hydroxyapatite (per ISO 13779) plasma spray coating is applied to the bony interface surfaces of the superior and inferior spinal plates.
The Mobi-C® Cervical Disc Prosthesis is indicated in skeletally mature patients for reconstruction of the disc from C3-C7 following discectomy at one level or two contiguous levels for intractable radiculopathy (arm pain and/or a neurological deficit) with or without neck pain, or myelopathy due to abnormality localized to the level of the disc space and at least one of the following conditions confirmed by radiographic imaging (CT, MRI, X-rays): herniated nucleus pulposus, spondylosis (defined by the presence of osteophytes), and/or visible loss of disc height compared to adjacent levels. The Mobi-C® Cervical Disc Prosthesis is implanted using an anterior approach. Patients should have failed at least 6 weeks of conservative treatment or demonstrated progressive signs or symptoms despite nonoperative treatment prior to implantation of the Mobi-C® Cervical Disc Prosthesis.
The Mobi-C® Cervical Disc Prosthesis should not be implanted in patients with the following conditions:
- Acute or chronic infection, systemic or at the operative site;
- Known allergy or sensitivity to the implant materials (cobalt, chromium, molybdenum, titanium, hydroxyapatite, or polyethylene);
- Compromised vertebral bodies at the index level due to previous trauma to the cervical spine or to significant cervical anatomical deformity or disease (e.g., ankylosing spondylitis, rheumatoid arthritis);
- Marked cervical instability on resting lateral or flexion/extension radiographs demonstrated by translation greater than 3.5mm, and/or > 11° angular difference to that of either adjacent level;
- Osteoporosis or osteopenia defined as DEXA bone mineral density T-score < -1.5;
- Severe facet joint disease or degeneration.
Warnings and Precautions
Warnings
- Mobi-C® should only be used by surgeons who are experienced with anterior cervical spinal procedures and have undergone hands-on training in the use of this device. Only surgeons who are familiar with the implant components, instruments, procedure, clinical applications, biomechanics, adverse events, and risks associated with the Mobi-C® should use this device. A lack of adequate experience and/or training may lead to a higher incidence of adverse events, including neurological complications.
-
Correct selection of the appropriate implant size is extremely important to assure the placement and function of the device. Information regarding proper implant size selection, implant site preparation, and the use of the instrumentation before, during, and after Mobi-C® surgery is provided in the Mobi-C® Surgical Technique Manual and the Mobi-C® Instrument System Instructions for Use. Users are advised to read and understand the surgical technique manual and instructions for use prior to surgery.
-
Due to the proximity of vascular and neurological structures to the implantation site, there are risks of serious or fatal hemorrhage and risks of neurological damage with the use of the device. Care must be taken to identify and protect these structures.
- Heterotopic Ossification (HO) is a potential complication associated with artificial cervical discs and could lead to reduced cervical motion. However, the presence of HO has not been correlated with adverse clinical outcomes involving the Mobi-C® Cervical Disc Prosthesis in the G050212 clinical trial.
Precautions
- The safety and effectiveness of this device has not been established in patients with the following conditions:
- Skeletally immature patients, pediatric or adolescent children (<21 years old), or those over the age of 67;
- Prior cervical spine surgery, including prior surgery at the index level;
- More than two diseased or immobile cervical spine levels requiring surgical intervention;
- Disc height less than 3mm measured from the center of the disc in a neutral position and disc height less than 20% of the anterior-posterior width of the inferior vertebral body;
- Significant kyphotic deformity or significant reversal of lordosis;
- Active malignancy;
- Paget’s disease, osteomalacia, or other metabolic bone disease;
- Taking medications known to potentially interfere with bone/soft tissue healing (e.g. steroids);
- Pregnancy;
- Diabetes mellitus requiring daily insulin management;
- Clinically extreme obesity (class III) as defined by the NIH Clinical Guidelines Body Mass Index (i.e. BMI >40);
- Neck or arm pain of unknown etiology;
- Systemic disease including AIDS, HIV, and Hepatitis;
- Intractable radiculopathy or myelopathy due to pathology at more than two levels and/or pathology not localized to the level of the disc space;
- Prior fusion at an adjacent vertebral level;
- Neck pain alone;
- Rheumatoid arthritis or other autoimmune disease;
- Neuromuscular disorders such as muscular dystrophy, spinal muscular atrophy, or amyotrophic lateral sclerosis;
- Acute mental illness or substance abuse.
Pre-operative, Intra-operative, and Post-operative
Pre-operative
- Patient selection is extremely important. In selecting patients for total disc replacement, the following factors can be of importance to the success of the procedure: the patient’s occupation or activity level, prior injury or other ongoing illness, alcoholism, or drug abuse; and certain degenerative diseases (e.g., degenerative scoliosis or ankylosing spondylitis) that may be so advanced at the time of implantation that the expected useful life of the device is substantially decreased.
- In order to minimize the risk of periprosthetic vertebral fractures, surgeons must consider all co-morbidities, past and present medications, previous treatments, etc. A screening questionnaire for osteopenia or osteoporosis, SCORE (Simple Calculated Osteoporosis Risk Estimation), may be used to screen patients to determine if a DEXA bone mineral density measurement is necessary. If DEXA is performed, the patient should be excluded from receiving the device if the DEXA bone density measured T score is < -1.5, as the patient may be osteoporotic or osteopenic.
- The patient should be informed of the potential adverse effects (risks/complications) contained in the insert (see ADVERSE EVENTS).
- Preoperative planning may be used to estimate the required implant size and to assure that the appropriate range of sizes is available for surgery. The procedure should not take place if the appropriate range of sizes will not be available.
- Examine all instruments prior to surgery for wear or damage. Instruments which have been used excessively may be more likely to break. Replace any worn or damaged instruments.
Intra-operative
- Use aseptic technique when removing the Mobi-C® from the innermost packaging. Carefully inspect each component and its packaging for any signs of damage, including damage to the sterile barrier. Do not use Mobi-C® implants if the packaging is damaged or the implant shows signs of damage.
- Use care when handling the Mobi-C® to ensure that it does not come in contact with objects that could damage the implant. Damaged implants are no longer functionally reliable. Visual inspection of the prosthesis assembly is recommended prior to implanting the device. If any part of the assembly appears damaged or not fully assembled, do not use.
- To prevent unnecessary damage to the bearing surfaces, ensure that tissue or other debris is not trapped within the device.
- The Mobi-C® should not be used with components or instruments of spinal systems from other manufacturers. See the surgical technique for step by step instructions.
- Surgical implants must never be re-used or reimplanted. Even though the device appears undamaged, it may have small defects and internal stress patterns that may lead to early breakage.
- Perform a complete discectomy of the disc space between the unci and up to the posterior ligament. Take care to release the foramen bilaterally. It is important to remove all anterior and posterior osteophytes on the superior and inferior vertebral endplates. Liberally cover bleeding with bone wax. To prevent weakening of the endplates, use of a burr is discouraged during endplate preparation. Use the Caspar Retractor as needed to maintain or modify distraction. Ensure proper alignment and placement of device components as misalignment may cause excessive wear and/or early failure of the device.
Post-operative
- Patients should be instructed in postoperative care procedures and should be advised of the importance of adhering to these procedures for successful treatment with the device including the avoidance of heavy lifting, repetitive bending, and prolonged or strenuous activity initially and for a period of weeks to months depending on the individual patient’s progress and the stability and functioning of the implant.
- Note to Physician: Although the physician is the learned intermediary between the company and the patient, the important medical information given in this document should be conveyed to the patient.
Additional Information
To request a paper copy of the Instructions for Use, contact HIGHRIDGE Spine Customer Service