InterGro® DBM
DBM in a Natural Carrier Designed to Provide Preferred Handling and Growth Factors.


Shaping the Future of DBM
The natural quality of the carrier and its containment and handling characteristics enable the surgeon to mold it to surgical sites, even in the presence of excessive fluids and under lavage.
System Features
InterGro DBM Fibers are composed of 100% Demineralized human cortical bone fibers and offer both osteoconductive and osteoinductive properties. Upon re-hydration the graft becomes flexible and moldable allowing it to be conveniently placed into bony voids or gaps that have been surgically created, or for filling osseous defects in non-weight bearing applications.

Biocompatibility
The carrier used in InterGro DBM is composed primarily of phosphatidylcholine, commonly referred to as lecithin. Lecithin is 100% natural, derived from soybeans, absorbed by the human body, and is key component of cell membranes.
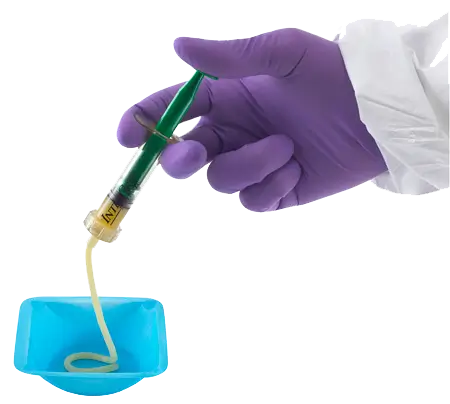
Preferred Handling
InterGro DBM is stored at room temperature and is available as a 40% DBM putty, 35% DBM paste, or a 35% DBM PLUS pre-mixed with Pro Osteon® 500R granules. Because InterGro DBM is non-water soluble, it resists lavage/irrigation.

Validated Osteoinductivity

Optimized Active DBM Content
Specifications
InterGro DBM Paste
0.5cc, 1cc, 2cc, 5cc 35% DBM content by weight.
InterGro DBM Putty
10cc 40% DBM content by weight.
InterGro DBM Plus (1mm–4mm Pro Osteon 500R granules are added)
2cc, 5cc, 10cc 35% DBM content by weight and premixed with resorbable coralline hydroxyapatite/calcium carbonate granules.
InterGro Fibers (100% DBM Fiber)
2cc, 5cc, 10cc, supplied in an open bore syringe.
Related Products
The HIGHRIDGE Spine Biologics portfolio encompasses a full range of bone graft materials to suit a variety of surgical needs.
Resources
Brochures
Additional Information
Contact Us
USA: 800 447-3625
To submit a complaint, please email SpineComplaints@highridgemedical.com
10225 Westmoor Dr. Westminster, CO 80021 USA
To obtain a copy of the current Instructions for Use (IFU) for full prescribing and risk information, please call 800 447-3625.
References
- Han B, Tang B, Nimni ME. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res. 2003;44(3–4):160–6.
- Han B, Tang B, Nimni ME. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J Orthop Res. 2003;21(4):648–54.
- Data on File. Internal Test Report TR-0428.
- Martin GJ Jr, Boden SD, Titus L, Scarborough NL. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine (Phila Pa 1976). 1999 Apr 1;24(7):637-45
Legal Manufacturer
Highridge Medical
10225 Westmoor Dr.
Westminster, CO 80021 USA

