- Paterson DC, Lewis GN, Cass CA. Treatment of delayed union and nonunion with an implanted direct current stimulator. Clin Orthop Relat Res. 1980 (148) may:117-128.
- Dunn AW, Rush GA. Electrical stimulation in treatment of delayed union and nonunion of fractures and osteotomies. Southern Medical Journal. 1984;77 (12):1530-1534.
- P.J. Cundy et. al. A Ten-Year Review of Treatment of Delayed Union and Nonunion with an Implanted Bone Growth Stimulator. Clin Orthop Relat Res. 1990 Oct;(259):216-22.
- Bodamyali, T., Kanczler, J.M., Simon,B., Blake,D.R., and Stevens,C.R. Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun, 1999. 264(3): p. 657-61.
- EBI® OsteoGenTM Surgically Implanted Bone Growth Stimulator Manual – PN 193102L.
- Data on file at Zimmer Biomet – P790005.
- EBI® OsteoGenTM Surgically Implanted Bone Growth Stimulator Surgical Technique Guide Procedures for Surgical Use – PN 193080L.
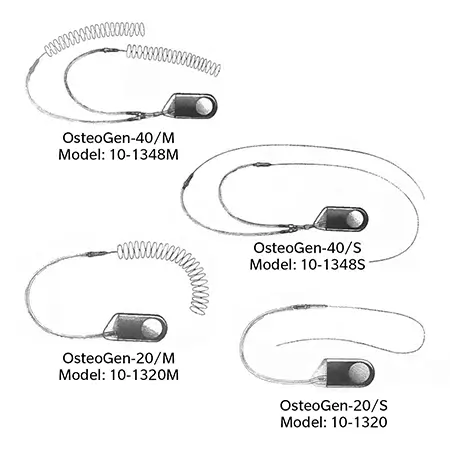
*OsteoGen Stimulator models currently not available.
†Healing of delayed union and nonunion was achieved in 72 of 84 patients. The time of injury to implantation was 3 months to 7.5 years which corresponds to the current definition of a nonunion i.e., a nonunion is considered to be established when there are no visibly progressive signs of healing. While osteotomy nonunions and pediatric use are not contraindicated, OsteoGen Stimulators do not have an approved indication to treat osteotomy nonunions or pediatric patients. The Osteostim S12 manufactured by Telectronics Pty. Ltd., Sydney, Australia utilized in the study was the predecessor to the EBI OsteoGen Surgically Implanted Bone Growth Stimulator.
††The original 1980 PMA included two clinical studies; neither was designed for long-term follow-up. In the first study (N=30) the patients were followed for a minimum of ten (10) years, with an overall follow-up rate of 48.2%. The long-term success rate was 66.7%. This calculation excludes the initial treatment successes not followed for ten (10) years (N=11). The second study (N=107) followed the patients for a minimum of four (4) years with overall follow-up rate of 25.6%. The long-term success rate was 38.8%. This calculation excludes the initial treatment successes not followed for four (4) years (N=58). In both studies, patients had difficult nonunion fractures: 0.7 and 1.5 number of average prior surgeries, average 28.4 and 24.3 months (median 24 and 16) disability since original injury, and 43.3% and 24.3% infected prior to treatment, respectively.
◊ While osteotomy nonunions are not contraindicated, OsteoGen Stimulators do not have an approved indication to treat osteotomy nonunions. All nonunions that had concomitant bone grafting healed with one exception, a synovial pseudarthrosis. Two other pseudarthrosis healed within two to three months respectively. Only one of three stimulators implanted without bone grafting produced union.
Δ While osteotomy nonunions are not contraindicated, OsteoGen Stimulators do not have an approved indication to treat osteotomy nonunions. The time of injury from initial surgery to implantation for the long-term review was less than 9 months to greater than 18 months which corresponds to the current definition of a nonunion i.e., a nonunion is considered to be established when there are no visibly progressive signs of healing.
**Although not indicative of human clinical results, outcomes from pre-clinical research have been implicated in various models of bone repair.
***EBI OsteoGen Surgical Implantable Bone Growth Stimulators are only approved for use with autograft. When the stimulators are used in conjunction with internal or external metallic fixation devices, caution must be taken to prevent contact with the cathode and the metallic implant. If the cathode comes into contact with the metallic implant, a current dissipation could occur. This reduction of current density could affect the level of therapeutic treatment normally expected. Secondly, if the cathode was to come into contact with the metal implant, possible corrosion or implant failure could occur at the site of contact.